BIOL2019FIGG35591 BIOL
The Impact of Early Developmental Stress on Inflammation in Adulthood
Type: Undergraduate
Author(s):
John Figg
Biology
Kelly Brice
Psychology
Chris Hagen
Biology
Claire Munster
Biology
Caitlyn Vilas
Biology
Advisor(s):
Michael Chumley
Biology
Gary Boehm
Psychology
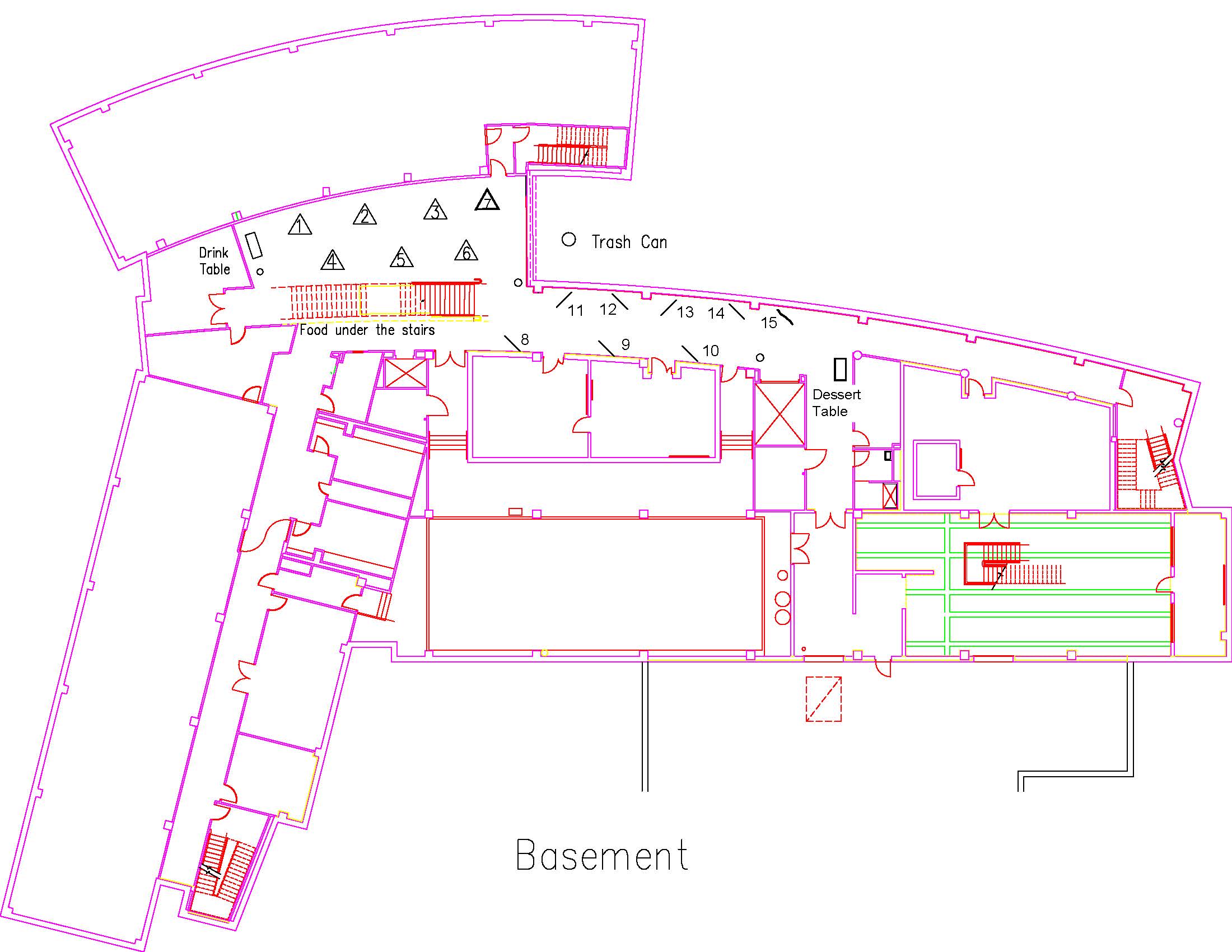
Location: Session: 1; Basement; Table Number: 10
(Presentation is private)Stress has been linked to altering acute and long-term inflammatory responses. Stress has been shown to activate inflammatory responses, specifically microglial activation in the brain. While acute inflammation is one of the first responses to fighting disease and infection, prolonged inflammation has been associated with neurogenerative disease such as Alzheimer’s disease. Stress at critical periods of development, known as early life stress (ELS) has been linked to chronic dysregulation of the hypothalamic-pituitary adrenal (HPA) axis, depression and alterations to microglial cells. The goal of this study is to investigate the effect of stress in mice during early development through maternal stress during pregnancy and the impact on neuroinflammation in adult offspring. In utero, offspring are vulnerable to the harmful effects of pro-inflammatory cytokines due to stress experienced by adult mice, following an ELS timeline. Three conditions were utilized: (1) mice undergoing stress during the entire pre-natal period and with the early post-natal period, (2) mice undergoing stress during the early postnatal period, and (3) mice undergoing no additional stress at any point. For mice in the combination-stress condition, there was an immunosuppressive effect through downregulation of pro-inflammatory cytokines. These data support existing publications that indicate an immunosuppressive role of prenatal stress, leaving the host less protected against chronic disease.
BIOL2019FINCH58955 BIOL
Investigating sex-based differences in pathogen resistance and immune responses in the fathead minnow (Pimephales promelas)
Type: Undergraduate
Author(s):
Miranda Finch
Biology
Lynsey Malin
Biology
Leah Thornton Hampton
Biology
Advisor(s):
Marlo Jeffries
Biology
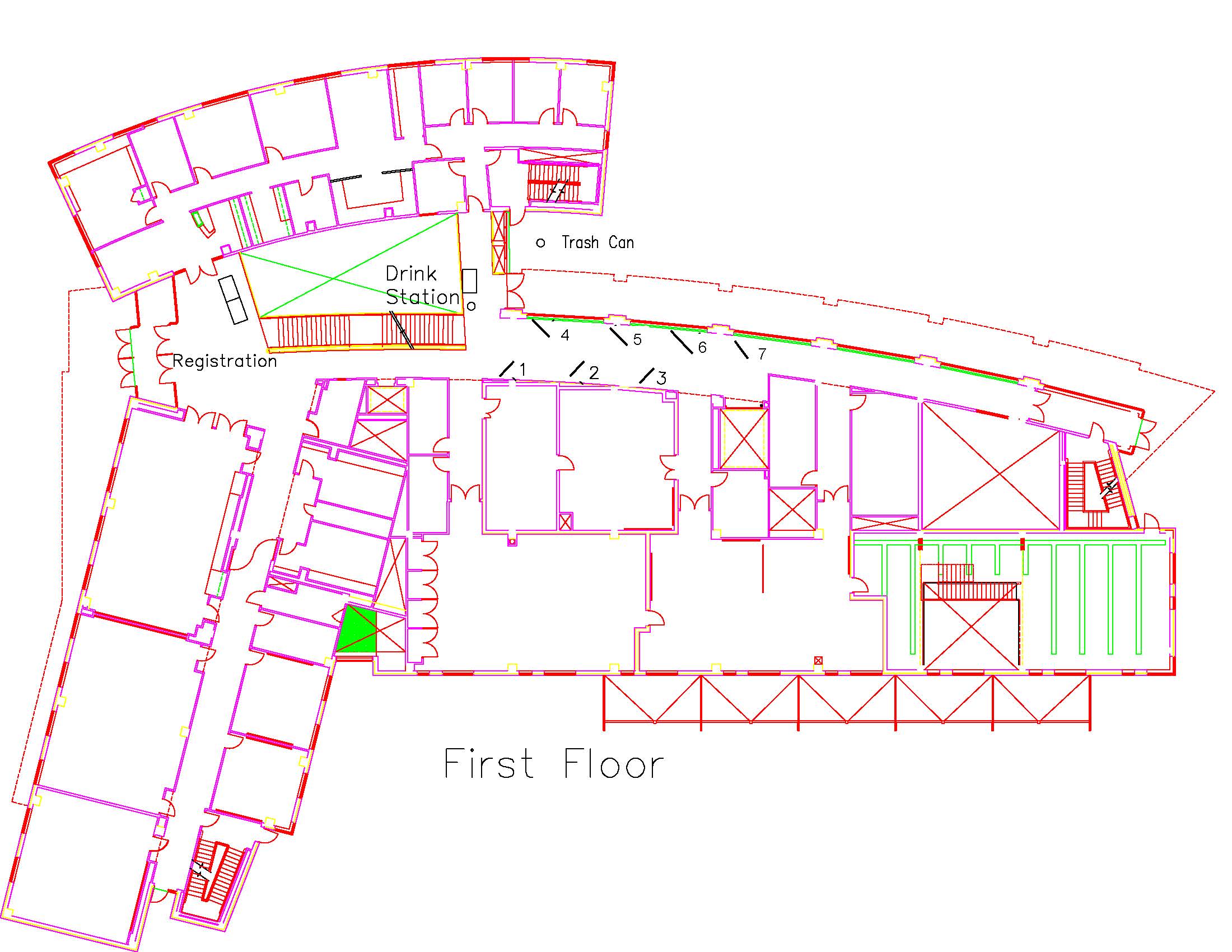
Location: Session: 1; 1st Floor; Table Number: 3
(Presentation is private)Males and females differ with regard to their immune response to a pathogen. Previous studies have observed males having reduced pathogen resistance. This suggests that they may be responding to pathogens differently. However, few studies have compared male and female immune responses following pathogen exposure. The purpose of this study was to examine sex-based differences in pathogen resistance and immune responses following exposure to a pathogen in adult fathead minnows (Pimephales promelas). To accomplish this, fish were bacterially infected with Yersinia ruckeri and the immune system’s ability to respond was monitored. Additionally, genes that are known to turn on during the immune response initiation were measured quantitatively providing insight into the molecular effect in minnows. At the whole organism level, male fish were less able to survive pathogen infection relative to female fish. At the tissue level, both male and female pathogen injected fish had decreased hematocrit percentages compared to the fish injected with a saline solution but did not differ from each other. At the molecular level, increased gene expression of interleukin 1β was seen in pathogen-injected males compared to pathogen-injected females and both sham-injected sexes indicating that pathogen-injected males mounted a larger inflammatory response at the molecular level. Taken together, this evidence suggests that the increased mortality observed among males earlier in the exposure to the pathogen may be due to the upregulated inflammatory response rather than the effects of the pathogen itself.
BIOL2019JORDAN10583 BIOL
Investigating the Role of Glymphatic Clearance of Amyloid Beta Through Exercise in C57BL/6J Mice
Type: Undergraduate
Author(s):
Rachel Jordan
Biology
Chris Hagen
Biology
Sofia Lopez
Biology
Julia Peterman
Psychology
Jordan White
Psychology
Advisor(s):
Michael Chumley
Biology
Gary Boehm
Psychology
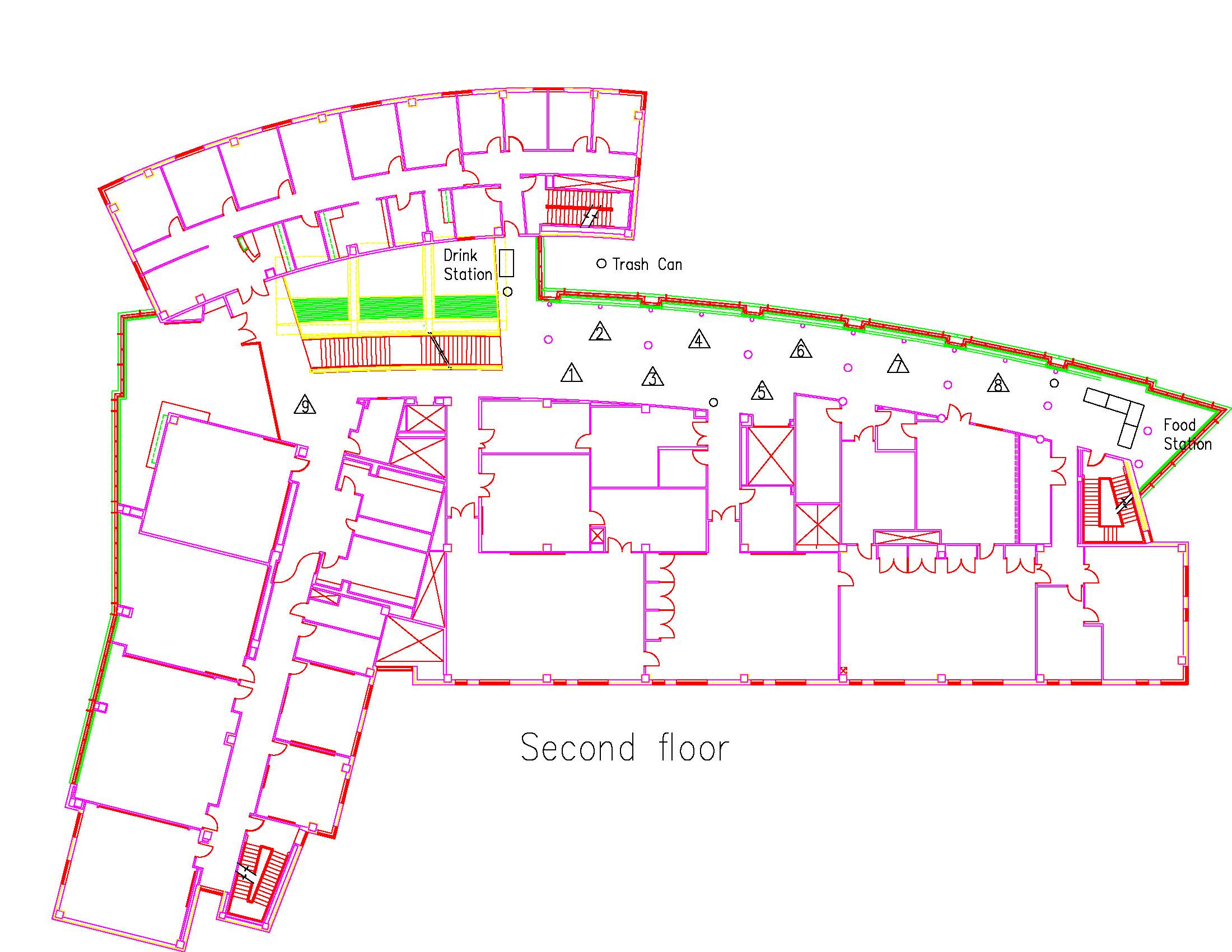
Location: Session: 1; 1st Floor; Table Number: 7

View PresentationAlzheimer’s disease (AD) is a very prevalent neurodegenerative disorder and is the 6th leading cause of death in the United States. Alzheimer’s disease (AD) is characterized by widely distributed amyloid plaques and neurofibrillary tangles, and is clinically associated with a progressive decline in memory and other cognitive functions. The major protein component of neuritic plaques is amyloid beta peptide. Several pieces of evidence have indicated that amyloid beta accumulates to form oligomeric states in the AD brain and cause the cognitive dysfunction commonly seen in patients. While a decrease in cognitive function is considered a hallmark of the disease, AD patients also exhibit decreased motor abilities and difficulties learning new motor tasks. Our lab’s previous investigations found voluntary running to decreased amyloid beta burden in C57/BL6 mice. The present experiment seeks to further explore the mechanism through which exercise induced amyloid beta clearance occurs. Previous studies have pointed to the function of the glymphatic system in the clearance of amyloid beta. The level and distribution of aquaporin 4 (AQP4) in the vascular endfeet of astrocytes is crucial to the normal function of the glymphatic system. Our experiment sought to determine a more detailed analysis of the role of AQP4 in the glymphatic clearance of amyloid beta. Using TGN-020, a selective AQP4 antagonist, we hope to further determine the importance of glymphatic clearance of amyloid beta in C57/BL6 mice through exercise. We hypothesize that mice receiving intraperitoneal TGN injections, thus blocking the function of AQP4, will experience decreased glymphatic clearance of amyloid beta. Understanding the process of amyoid beta clearance can aid in treatments for both the pathological and clinical affects associated with AD.
BIOL2019LOSEFSKY29412 BIOL
The role of ClpX and ClpP in antibiotic resistance in Bacillus antracis
Type: Undergraduate
Author(s):
Quinn Losefsky
Biology
Advisor(s):
Shauna McGillivray
Biology
Location: Session: 1; 2nd Floor; Table Number: 4
View PresentationBacillus anthracis is a Gram-positive bacterium that causes anthrax in humans. It is a significant microorganism in that many proteins important to virulence or pathogenesis are highly conserved in many other pathogenic bacteria. Our lab has previously identified the protein ClpX in Bacillus anthracis as metabolically significant in antibiotic resistance. Specifically, B. anthracis lacking the ClpX gene (ΔClpX) are significantly more susceptible to antibiotics that target the bacterial cell wall such as penicillin than the wild type. ClpX has multiple functions; primarily it interacts with ClpP to form a proteolytic complex that degrades dysfunctional or obsolete proteins. ClpX also has an independent chaperone function, moving proteins around the cell. This project has focused on determining if the pathway of decreased antibiotic resistance in mutant B. anthracis is dependent on ClpX interactions with ClpP, or if ClpX can function independently. To test this, a point mutation (I265E) was made in the ClpX gene at the site that has been previously identified as the site of interaction between ClpX and ClpP in Staphylococcus aureus. The ClpX genes in B. anthracis and S. aureus exhibit a high degree of conservation particularly in this region, and it is expected that this site will also be critical for ClpX and ClpP interaction in B. anthracis. The mutated ClpX gene (I265E) has been confirmed with sequencing and has been transformed as an inducible expression plasmit into the ΔClpX B. anthracis strain. The next step is to perfom growth assays in antibiotics such as penicillin to ascertain if the mutated expression vector can restore antibiotic resistance to ΔClpX.
BIOL2019MOROTECOSTAS54682 BIOL
The Effect of BRCA1 Activation on Protein Structure and Function
Type: Undergraduate
Author(s):
Brian Morote-Costas
Chemistry & Biochemistry
Advisor(s):
Mikaela Stewart
Biology
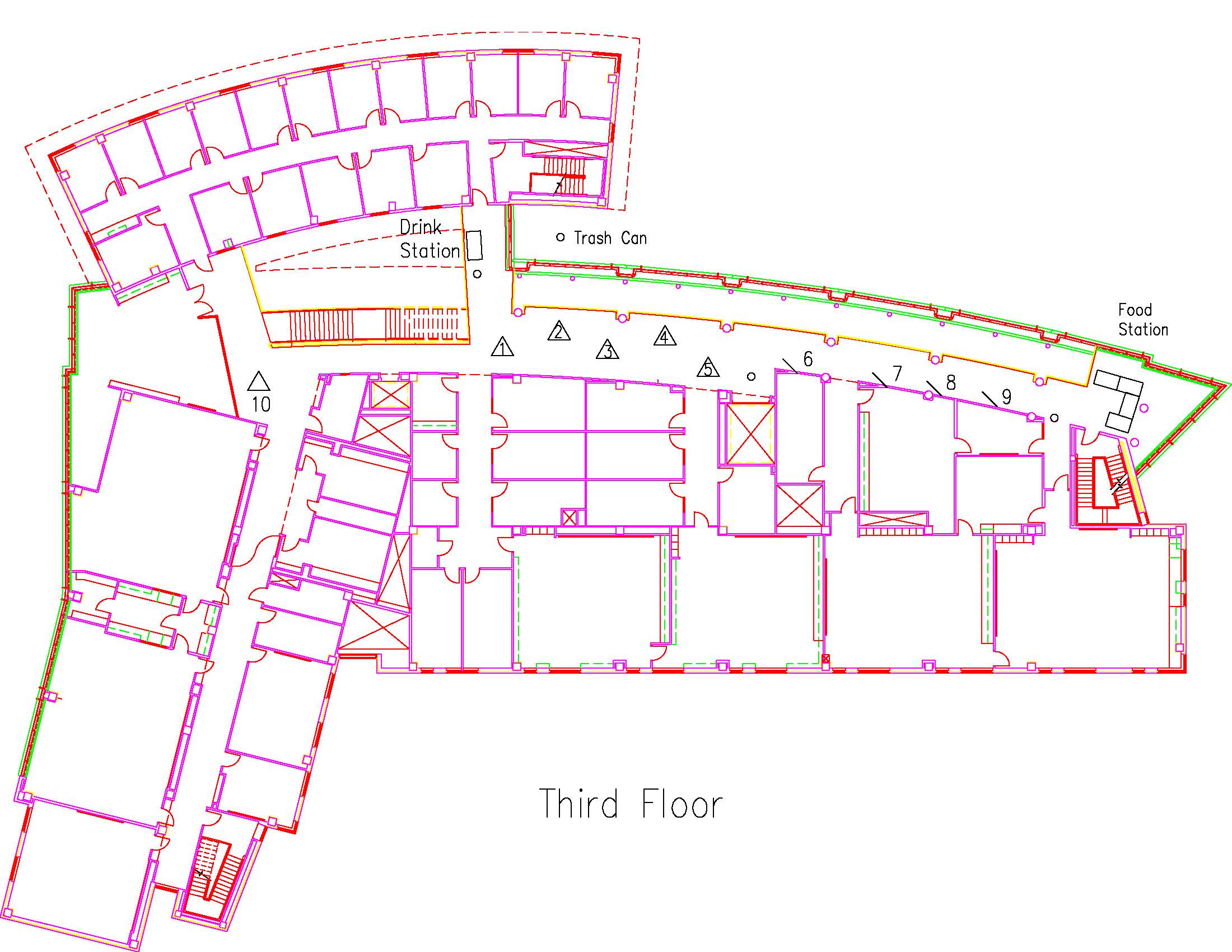
Location: Session: 1; 3rd Floor; Table Number: 3
View PresentationThe proper function of the Breast Cancer susceptibility gene 1 (BRCA1) and its subsequent protein in the human body is vital to healthy individuals. The malfunction of BRCA1 due to a mutation is associated with an increased risk of breast or ovarian cancer of up to 80%. The key to understanding whether a mutation in BRCA1 will lead to higher risk of cancer is its location. Many of its interactions with other proteins take place in the central region of BRCA1. Currently, little is known about how mutations located in this region affect BRCA1 structure and function. The central region is where BRCA1 interacts with another tumor suppressor called PALB2 to perform DNA repair. The central region site studied in this project can be activated as a response to DNA damage, influencing BRCA1 and PALB2 interaction to generate a damage repair response. We show how activation of BRCA1 affects its structure and function on the molecular level. The accomplish this we created three mutations in the central region that mimic activation of BRCA1 to identify possible significant changes in protein-protein interactions using biochemistry and structural biology techniques like Isothermal titration calorimetry and circular dichroism.