CHEM2021SHERMAN26246 CHEM
Practical Synthesis of Alkenyl Phosphorus Compunds
Type: Undergraduate
Author(s):
Emily Sherman
Chemistry & Biochemistry
Advisor(s):
Jean-Luc Montchamp
Chemistry & Biochemistry
Benjamin Janesko
Chemistry & Biochemistry
Anne VanBeber
Nutritional Sciences
Location: Zoom Room 2, 03:27 PM
View PresentationAlkenyl phosphorus compounds appear in multiple industrial products, from flame retardants to fungicides. Although several methods are available to synthesize these compounds, many require expensive catalysts, inaccessible starting materials, or multi-steps sequences. In response to these issues, this project sought to develop an efficient, two-step method to synthesize alkenyl phosphorus compounds from simple ketones. We compare acid and base catalysts and find both are effective in the first reaction step; furthermore, a one-pot reaction provides comparable yields to the reactions conducted with a purified intermediate. These findings lay the foundation for the exploration of more complex substrates, including those utilized in industrial applications.
CHEM2021VEALS38707 CHEM
Synthesis and Characterization of an Iodo-substituted Macrocyclic Complex: Comparison of Pyridine Modification
Type: Undergraduate
Author(s):
Diandria Veals
Chemistry & Biochemistry
Advisor(s):
Kayla Green
Chemistry & Biochemistry
Location: Zoom Room 1, 03:03 PM
View PresentationPyridine macrocycles have useful applications due to their ability to complex with metals. A library of substituted pyridine macrocycles exists along with how modifications at Carbon 4 impact compound reactivity. Despite literature about similar pyridine macrocycle structures, little is known about how an iodo-substituted pyridine macrocycle will alter the properties of the compound when complexed to Copper. To understand the fundamental characteristics of an Iodo-substituted pyridine macrocycle, the ligand is synthesized followed by electronic environment analysis via 1H NMR. Ultraviolet-Visible Spectroscopy is used to verify ligand complexation with Copper (II) metal followed by X-ray diffraction to determine metal binding nature of the complex. Cyclic Voltammetry analysis is used to support the theory that the iodo functional group behaves as an electron withdrawing group. This compound serves as a comparison to explain the results of the Chloro-substituted pyridine macrocycle as well as a gateway molecule for the synthesis of new pyridine macrocycles.
CHEM2020BUDENSIEK51413 CHEM
Fabrication and Characterization of Sub-Micron Plant-Derived Silicon Nanoparticles for Drug Delivery
Type: Undergraduate
Author(s):
Hailey Budensiek
Chemistry & Biochemistry
Advisor(s):
Jeffery Coffer
Chemistry & Biochemistry
View PresentationPorous silicon nanoparticles exhibit great potential as drug delivery vectors due to their high surface-area-to-volume ratio allowing for increased efficacy of surface functionalization and therapeutic loading capabilities. This data set demonstrates the fabrication of a class of plant-derived materials which are sub-micron in size and capable of functionalization with primary amine groups through the addition of APTES.
The production of porous silicon particles (pSi) is achieved through magnesiothermic reduction of silica containing Tabasheer powder isolated from the nodal joints of the Bambuseae plant. Efficacy of this reduction is evaluated using techniques including X-ray diffraction and Energy-dispersive X-ray spectroscopy which show successful reduction of silica starting material to porous silicon.
High energy ball milling followed by reduction is used to produce pSi particles of sub-micrometer size while also allowing for a significantly higher yield (~90%) of material than previous methods. Particle size is confirmed via electron microscopy and dynamic light scattering (DLS).
Following reduction, surface functionalization of silicon nanoparticles with primary amine groups was carried out using a 4% (v/v) solution of APTES in acetone. The evaluation of this functionalization was conducted using techniques including zeta potential and infrared spectroscopy (IR). Zeta potential values are found to be approximately -10 mV. This data demonstrates successful amino silanization.
The results achieved through these methods suggest successful fabrication of pSi nanoparticles and subsequent functionalization for future use as a drug delivery vector.
CHEM2020GOEHRING48218 CHEM
Creating Biocompatible Polymers Loaded With Porous Silicon Potentially For Drug Delivery
Type: Undergraduate
Author(s):
Lexi Goehring
Chemistry & Biochemistry
Advisor(s):
Jeff Coffer
Chemistry & Biochemistry
View PresentationDrug delivery is the process by which medications are administered to the body. This is complex due to the difficulty of determining compounds that have the proper biocompatibility and permissibility to our human cells. Many medications are taken orally; however, there are advantages to administering medication subcutaneously or by inserting it in the inner corner of the eye. Porous films made out of biocompatible polymers provide a good platform for drug delivery as they have the ability to be loaded with plant derived porous Silicon. Functionalizing the porous silicon using (3-aminopropyl)triethoxysilane and glutaraldehyde can be done in an attempt to covalently attach particles to the film which is important for embedding them into the pores of the film. Porous silicon has biocompatible properties and can be loaded with drugs then modified to alter the release of those drugs in the body. This method has the potential to be a useful drug delivery method due to the biocompatible and biodegradable properties of the material and the ability to manipulate the material in order to maximize drug release.
CHEM2020LE35772 CHEM
POROUS SILICON NANOTUBES AS POTENTIAL VECTORS FOR SMALL INTERFERING RNA DELIVERY
Type: Graduate
Author(s):
Nguyen Le
Chemistry & Biochemistry
Advisor(s):
Jeffery Coffer
Chemistry & Biochemistry
Giridhar Akkaraju
Biology
(Presentation is private)In cancer therapy, nucleic acid-based therapeutic strategies have been extensively investigated to suppress mutated gene expression, thereby inhibiting cancer cell growth. Among the approaches, small interfering (siRNA)-mediated gene silencing has been envisaged as a promising therapeutic approach to silence specific gene expression by targeting mRNA of the unwanted gene for degradation, thereby readily controlling cellular functions. However, delivery of small interfering RNA (siRNA) has been known to encounter multiple challenging barriers, such as blood circulation and cellular internalization, thus limiting the potential merits of this therapeutic strategy. While non-viral vectors have been preferred owing in part to better immune system compatibilities, porous silicon (pSi) with various geometric shapes (e.g. platelet and discoid) have recently been demonstrated as exceptional delivery carriers of siRNA in various disease models. Here our initial in vitro studies show that silicon in a unique one-dimensional porous nanotube structure (pSiNTs) can serve as a promising vector for delivery of siRNA to limit target gene expression, thereby expanding the library of possible nanostructures of Si in delivery of siRNA.
In this work, we demonstrate that pSiNTs after being functionalized with 3-(aminopropyl)triethoxysilane (APTES) can deliver enhanced green fluorescence protein (EGFP)-targeting-siRNA via electrostatic conjugation and suppress EGFP expression in HeLa cervical cancer cells by up to 50%. Cytocompatibility and biodegradation of the functionalized pSiNT matrix upon siRNA delivery are characterized by ATP quantification assays (CellTiter Glo) and Transmission Electron Microscopy imaging (TEM) respectively. These results encourage further development of pSiNTs in therapeutic applications.
CHEM2020SEGURA63908 CHEM
Synthesizing a vaccine for the treatment of addiction to the fentanyl opioid
Type: Undergraduate
Author(s):
Carolina Segura
Biology
Advisor(s):
Jean-Luc Montchamp
Chemistry & Biochemistry
View PresentationThe objective of this project is to make a vaccine that will negate the effects of the powerful opioid fentanyl in the long term. Fentanyl is a strong synthetic opioid that is 50 to 100 times more potent than morphine. According to the CDC, there were over 70,000 deaths due to street drug overdoses, which has increased in the last ten years. 40 % of these deaths are related to fentanyl overdoses, therefore it is imperative that approaches are developed to combat this alarming increase in deaths. The vaccine against fentanyl will be synthesized out of molecules that will take advantage of fentanyl’s amide functional group to be hydrolyzed into safe byproducts. Any patient that is administered with the vaccine, will not feel the effects of the opioid because the immune system will hydrolyze the drug as soon as it enters. This project will exploit the properties of both catalytic antibodies (CAbs) and transition state analogs. The Cabs will trigger an immune response to attract phagocytic cells, such as macrophages to phagocytose pathogens and eliminate them from the system. However, if the molecule resembles the transition-state of fentanyl hydrolysis, then the antibodies can cleave the fentanyl in a fast and efficient manner due to their catalytic properties. Therefore, after immunization, a person who is addicted to fentanyl would no longer feel the effects of the opioid because it will be degraded as an immune response is triggered, creating a long-term possible solution to one factor of the “opioid crisis.”
CHEM2019BEERI11498 CHEM
Using Surface Polymer Networks to Connect DSPEC Components for a High Solar Energy Conversion Efficiency
Type: Graduate
Author(s):
Debora Beeri
Chemistry & Biochemistry
Advisor(s):
Benjamin Sherman
Chemistry & Biochemistry
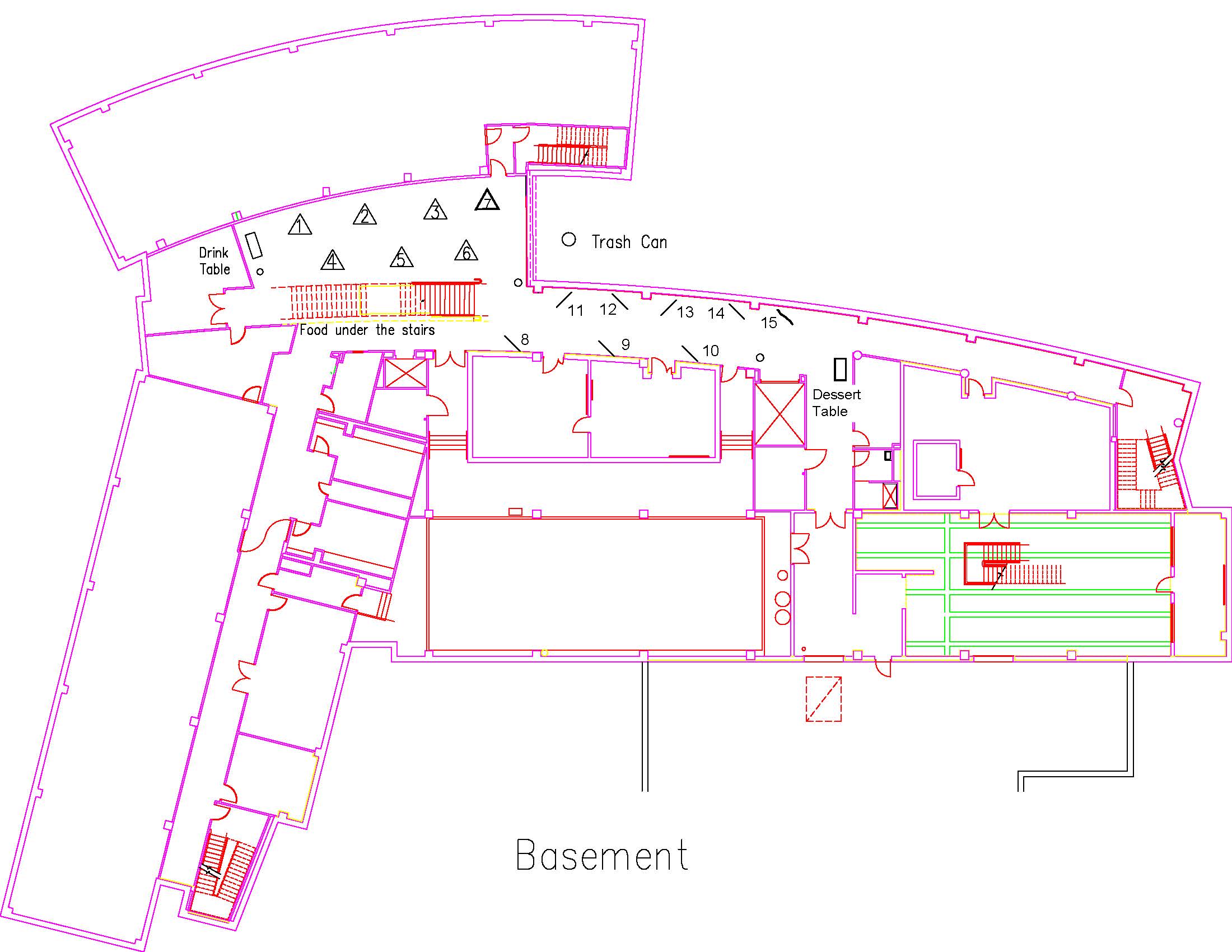
Location: Session: 1; Basement; Table Number: 5
View PresentationIt is extremely important in our age to look for alternative, more environmentally favorable energy sources. The Sun is a largely unused and widely available energy source to power human industry which can be utilized in different ways. Photovoltaic cells directly convert solar energy to electricity but only provide power when illuminated. Supplying solar-sourced energy during night hours and inclement weather requires conversion to another form, for instance into chemical fuel by means of water splitting into oxygen and hydrogen. This strategy, inspired by natural photosynthesis, is currently a promising and actively researched approach. However, achieving a high energy conversion efficiency, which is essential for industrial implantation of the method, remains a primary goal.
A Dye-Sensitized Photoelectrochemical Solar Cell (DSPEC) is specifically designed for using solar energy to generate hydrogen from water. We are pursuing the formation of photoanodes with polymer surface coatings prepared by electropolymerization. The polymer interfaces are designed to promote directional electron transfer at the interface, thereby resulting in a better solar energy conversion efficiency. The structure of the surface polymer enables the incorporation of catalyst units to the interface. To this end, we have prepared several novel iridium-oxide nanoparticle suspensions, using two different synthetic methods, to serve as the water-oxidation catalysts in our system. During the synthesis, the nanoparticles are functionalized with specific capping groups that contain terminal double bonds, through which they can be incorporated to the surface polymer electrochemically. Using acrylic acid and acrylamide as small molecule precursors, electro-polymer coatings have been prepared on FTO (fluorine-doped tin oxide) surfaces. Future research work will involve the incorporation of functionalized iridium oxide nanoparticles in the poly(acrylic acid/acrylamide) films and the characterization of their catalytic activity toward water oxidation. The method will then be extended to tin-oxide and titanium-dioxide semiconductor electrodes for preparing photo-active interfaces.
CHEM2019BLITCH45678 CHEM
Strong Hydrogen Bonds to Weak Bases: An Orbital Overlap Perspective
Type: Undergraduate
Author(s):
Alexandra Blitch
Chemistry & Biochemistry
Advisor(s):
Benjamin Janesko
Chemistry & Biochemistry
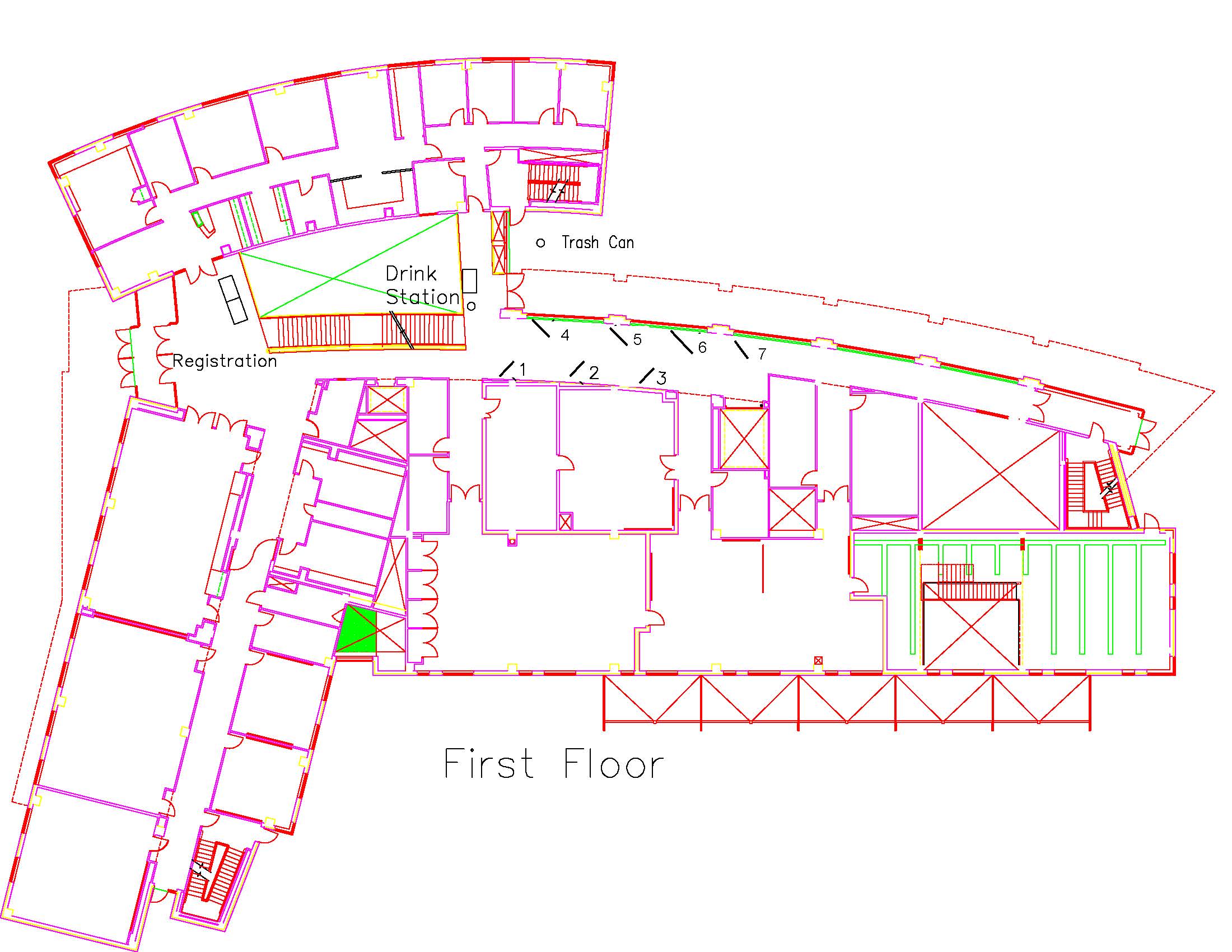
Location: Session: 2; 1st Floor; Table Number: 7
View PresentationIon solvation is fundamental in biochemistry. It controls the biophysical processes of protein solubility, reactivity, phase separation, crystallization and informational equilibria involving proteins and polypeptides. Ion solvation depends on the solute-solvent interactions which are governed by the properties of solvent like polarity, hydrogen bonding and ability to donate or accept electrons. These properties are subject to Pearson’s hard–soft acid–base (HSAB) effect and are characterized as hardness and softness of solvents. There have been attempts to connect the solvent hardness-softness to molecular properties and some empirical scales have been devised like μ-scale, DS scale and difference between the IR wavenumber shift of the C-I stretch of ICN and the O-H stretch of phenol. Only limited attempts have been reported to correlate the properties of solvents obtained from quantum chemical calculation to these empirical scales of solvent hardness-softness.
Our new quantum chemical descriptor, Orbital Overlap Distance, D(r), measures the size of orbital lobes that best overlap with the wavefunction around an atom. Compact, chemically stable atoms in the molecule tend to have overlap distances smaller than chemically soft, unstable atoms. Plots of D(r) on computed molecular surfaces, like electron density or spin density, distinguishes and quantifies the chemically soft and hard regions of a molecule. We propose that D(r) can be considered in terms of HSAB theory in order to predict solvation of ions. Our initial studies exhibit that D(r) of many common solvents correlates well with Marcus’s empirical μ-scale of solvent softness. Our studies provide a direct method to estimate the softness-hardness of solvents by using standard quantum chemical calculations.
CHEM2019BUCKINGHAM30657 CHEM
The Effects of Microgravity on the Creation of Nylon 6-10
Type: Undergraduate
Author(s):
Allison Buckingham
Chemistry & Biochemistry
Keira Clotfelter
Chemistry & Biochemistry
Jack Dietz
Biology
Tommy Gifford
Chemistry & Biochemistry
Waylan Kisor
Chemistry & Biochemistry
Advisor(s):
Magnus Rittby
Chemistry & Biochemistry
Location: Session: 2; Basement; Table Number: 9

View PresentationThis is experiment is designed to test how Nylon 6-10 is constructed and responds in a microgravity environment. Nylon 6-10 is a very flexible fiber. It consists of two chemicals called polypropylene and sebacoyl chloride to make the nano-structure for Nylon 6-10. We have developed several of ideas on what will happen to Nylon 6-10 in micro-gravity. We think that it will change the molecular structure of the Nylon 6-10 in micro-gravity for the better or worse. The good variable is that Nylon 6-10 might change into a very flexible, durable substance for many different applications both on Earth and in space. One concern we have is that Nylon 6-10 might change the molecular structure to not form any fibers or it might not dry by absorbing air molecules.
We decided to use Nylon 6-10 because of its overall construction. The industrial process for Nylon 6-10 is stronger and more flexible than Nylon 6-6. It is basically liquid rope. It can be used for repairs and manufacturing. It is an industrial chemical. A variety of products are created using Nylon 6-10, toothbrushes, paint brushes and even your underwear. It is a very common product in many of different industries and is a very useful product. It behaves like nylon fiber for thread or can be used for manufacturing different tools such as epoxy or fiberglass. The industrial ideas are very extensive and there are many suppliers.
CHEM2019CAREY22340 CHEM
Synthesis of Heterodimeric Macrocycles
Type: Undergraduate
Author(s):
Hannah Carey
Chemistry & Biochemistry
Jason Mars
Chemistry & Biochemistry
Advisor(s):
Eric Simanek
Chemistry & Biochemistry
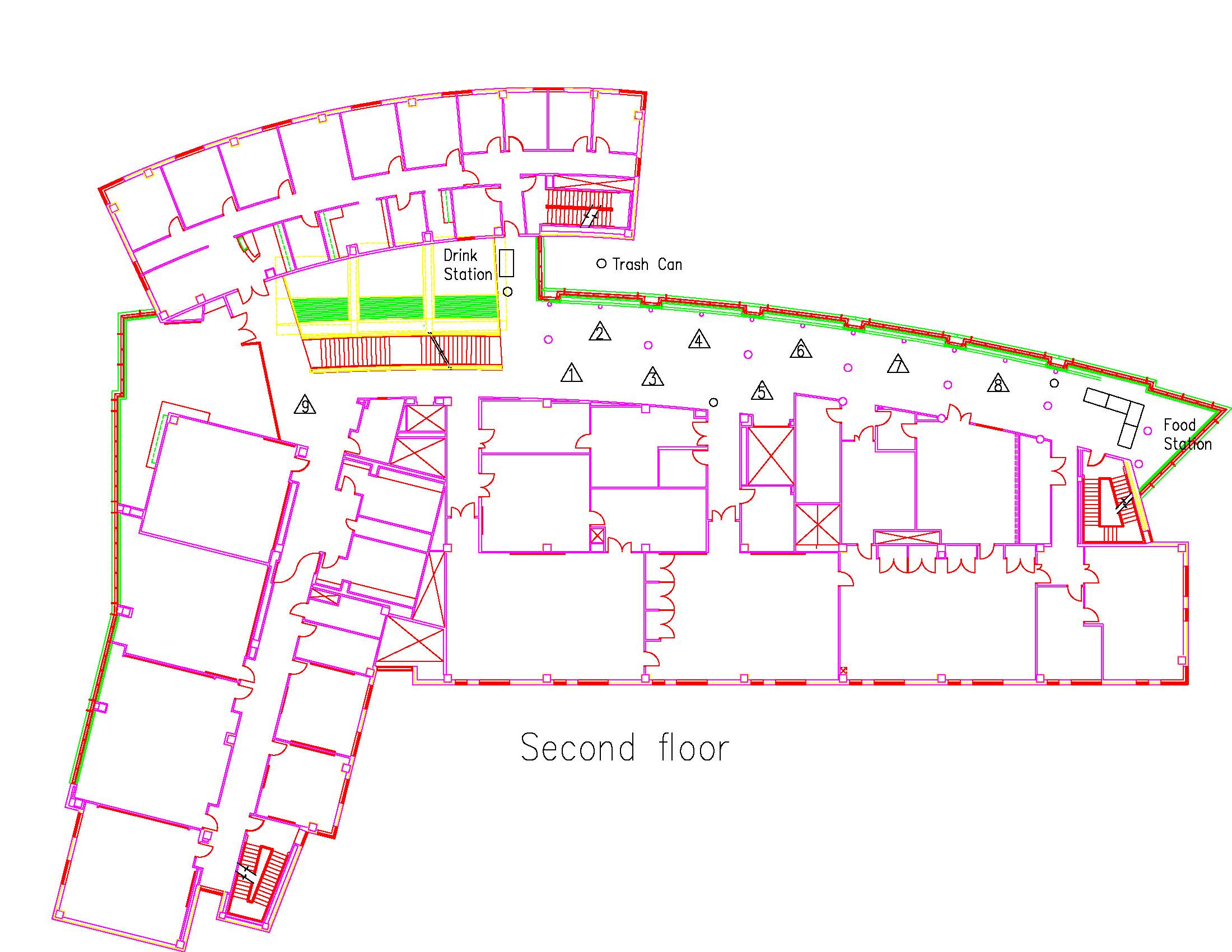
Location: Session: 1; 2nd Floor; Table Number: 5
(Presentation is private)Recent trends in drug discovery research are directed at targeting protein-protein interactions. Blocking these interactions could be an effective strategy for treatment. Here, the synthesis of a macrocycle, a large ring-shaped molecule that is the same size as many protein-protein interaction sites, is described. The synthesis relies on the preparation of two different, crescent-shaped molecules through short, multistep syntheses. When these two molecules are combined together and subjected to acid to reveal reactive groups, a spontaneous assembly process occurs. The macrocycle is characterized by conventional methods including 1H NMR (which reveals a diagnostic signal for cyclization), 13C NMR, and mass spectrometry.
CHEM2019CASTILLO16416 CHEM
RATIOMETRIC MOLECULAR ROTORS FOR DETERMINING PHASE-TRANSITIONS OF SOFT MATERIALS
Type: Graduate
Author(s):
Marlius Castillo
Chemistry & Biochemistry
Zygmunt Gryczynski
Physics & Astronomy
Zhangatay Nukureyev
Physics & Astronomy
Advisor(s):
Sergei Dzyuba
Chemistry & Biochemistry
Location: Session: 1; Basement; Table Number: 3

(Presentation is private)Soft matter, such as organogels, waxes and polymer films have found numerous applications in various areas of sciences, engineering and medicine. Ability to assess and monitor their structural organization and physical properties is of the outmost importance. However, there are no convenient methods to accomplish this task.
Small molecule environmental probes have been instrumental in providing information about changes of various types of media upon exposure to external stimuli. Our group has demonstrated the validity of using these probes, also known as molecular rotors, for investigating various types of media. This poster will highlight our efforts on the developments and applications of ratiometirc molecular rotors that allow determining structural integrity as well as properties of various industrially important, medically- and energy-relevant soft matter materials.
CHEM2019CLARK13102 CHEM
Synthesis of Pancratistatin and similar analogs
Type: Undergraduate
Author(s):
Brian Clark
Chemistry & Biochemistry
Advisor(s):
David Minter
Chemistry & Biochemistry
Location: Session: 1; 1st Floor; Table Number: 6

View PresentationPancratistatin is a natural alkaloid that can be isolated from the bulbs of Hymenocallis littoralis, which is a tropical plant commonly referred to as the Spider Lily. Pancratistatin has been shown to have potent cytotoxic anti-tumor activity in biological testing, meaning that it could be a key component for designing natural anti-cancer drugs. The key structural component responsible for the cytotoxic activity of Pancratistatin is the phenanthridone ring system. Pancratistatin has also been proven to combat RNA-containing flaviviruses such as Yellow Fever, Zika, and West Nile Virus. Previously reported procedures for synthesizing Pancratistatin have been reasonably successful, but they all involve the use of lengthy sequences that produce low yields in order to reach the desired product. The purpose of this research project is to provide a more efficient synthesis by increasing the final yield and decreasing the number of steps required. Through successfully synthesizing Pancratistatin, several different analogs of the molecule that contain the phenanthridone ring will also be obtained.
CHEM2019EBER15332 CHEM
Synthesis of 1,1’-Dideaza-Quinine: A Proof of Concept
Type: Undergraduate
Author(s):
Jackson Eber
Chemistry & Biochemistry
David Minter
Chemistry & Biochemistry
Adam Montoya
Chemistry & Biochemistry
Advisor(s):
David Minter
Chemistry & Biochemistry
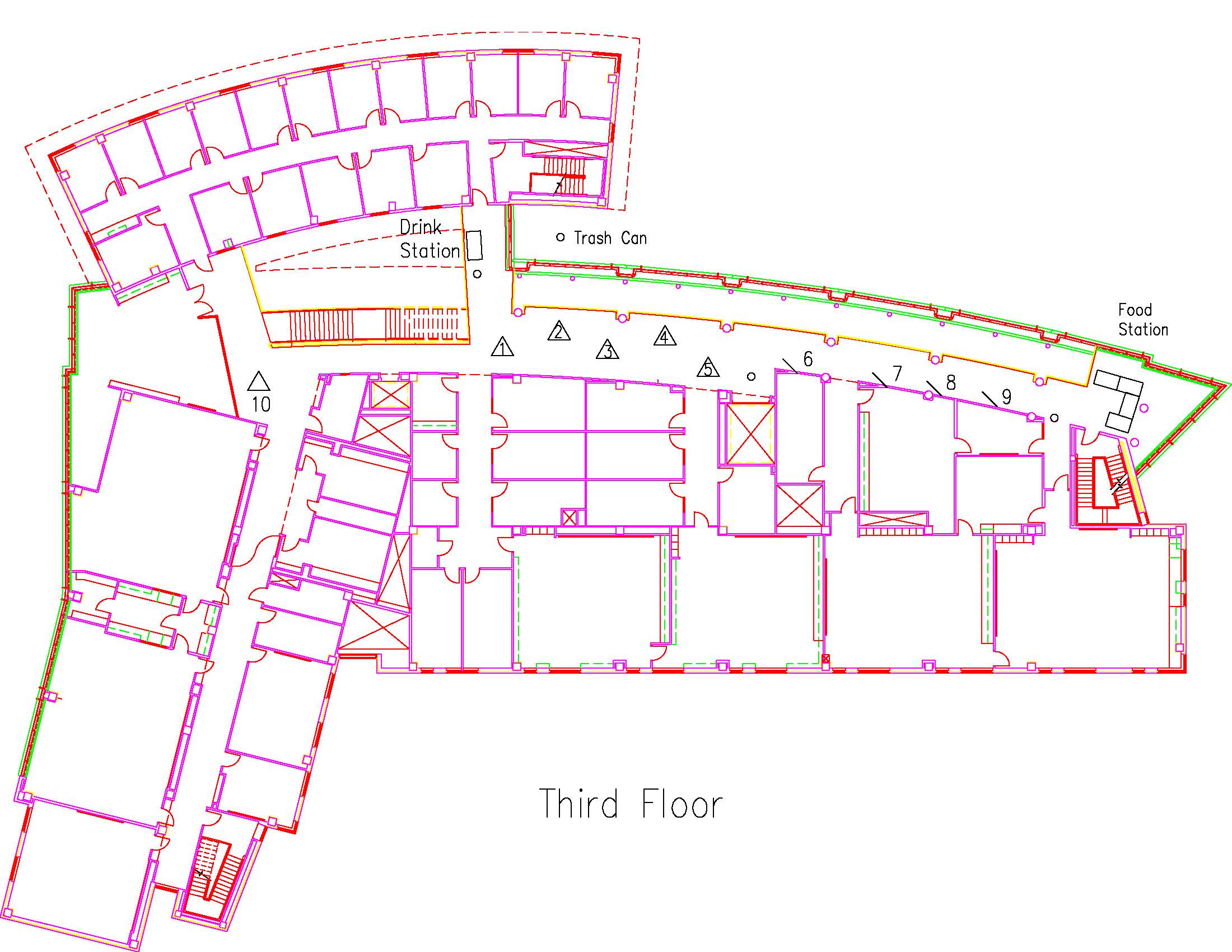
Location: Session: 1; 3rd Floor; Table Number: 3
View PresentationQuinine is a naturally occurring alkaloid found in the bark of the cinchona tree.1 Its medicinal relevance cannot be overstated as it is one of the most widely used anti-malarial drugs in the world.1 While the synthetic pathway to derive quinine is of limited relevance due to its abundance and ease of extraction, the puzzle of engineering reactions to isolate a stereochemically pure product of quinine captivated chemists for generations. The purpose of this study was to prove the conceptual route proposed by Stotter, Friedman, and Minter2 for the stereochemically pure total synthesis of quinine via a non-nitrogenous analog where the two nitrogen atoms of quinine are substituted with carbon atoms. The product of the analogous route is 1,1’-Dideaza-Quinine. Quinine is stereochemically complex, containing four separate stereocenters, thus the synthesis of quinine opens up the possibility of generating sixteen different isomeric structures.3 While the total synthesis of quinine with the correct stereochemistry was accomplished in 2001,3 the proposed route simplifies the process by relying on a stereospecific aldol condensation to eliminate potential isomerization.2 The results of the study validate the proposed route and add to the field of Organic Synthesis by illustrating an example of a stereoselective aldol condensation. Additionally, due to the analogous nature of the synthetic route utilized, many novel compounds were generated adding to the body of knowledge available to the Chemistry community.
CHEM2019FAHIM64637 CHEM
Unusual Liquid-liquid Phase Separation of Lysozyme Aqueous Solutions at Physiological pH and Salt Concentration
Type: Graduate
Author(s):
Aisha Fahim
Chemistry & Biochemistry
Advisor(s):
Onofrio Annunziata
Chemistry & Biochemistry
Location: Session: 1; 3rd Floor; Table Number: 1

View PresentationLiquid-liquid phase separation (LLPS) of protein aqueous mixtures is the reversible condensation of protein-rich micro droplets occurring below a well-defined LLPS temperature. LLPS studies of protein mixtures are fundamental for understanding the membrane-less compartmentalization inside living cells, protein-aggregation diseases, protein-based drug formulations, enzyme-based materials and molecular interactions. It is known that aqueous solutions of the protein lysozyme in the presence of phosphate buffer at neutral pH and physiological salt concentration undergo LLPS upon cooling below ≈ 0 °C. The obtained lysozyme-rich micro droplets rapidly dissolve upon heating above the LLPS temperature. In this work, it will be shown that an apparently undisruptive substitution of phosphate buffer with another well-known buffer, 4-(2-hydroxyethyl)-1-piperazineethanesulfonate (HEPES), to lysozyme aqueous solutions significantly alter the LLPS mechanism. Specifically, contrary to the case of phosphate buffer, the micro droplets produced below ≈ 0 °C remain surprisingly stable upon heating even at ≈ 30-40 °C. Related LLPS studies in both acidic and basic conditions show similar anomalous LLPS behavior. Our results indicate that HEPES triggers a second protein self-assembly process that is catalyzed by LLPS. These findings show that protein aqueous mixtures in the presence of HEPES buffer could be exploited for the preparation of protein-based materials. They also suggest that the combination of a protein self-assembly with LLPS may be a mechanism involved in the formation of membrane-less globular compartments inside the cytoplasm of living cells.
CHEM2019HENDERSON2011 CHEM
Chemical hardness and orbital overlap in substituted aromatics
Type: Undergraduate
Author(s):
Nicholas Henderson
Chemistry & Biochemistry
Arshad Mehmood
Chemistry & Biochemistry
Advisor(s):
Benjamin Janesko
Chemistry & Biochemistry
Location: Session: 2; Basement; Table Number: 5

View PresentationHard-soft acid base theory is often used to explain the selectivity of chemical reactions, under the assumption that hard (soft) nucleophiles prefer to react with hard (soft) electrophiles. Computationally, quantifying the relative hardness and softness of different sites in a molecule remains challenging. Our "orbital overlap distance function" allows us to quantify which regions in a molecule contain compact vs. diffuse molecular orbitals. Here we explore the idea that compact molecular orbitals correspond to chemically hard regions, and that diffuse and polarizable orbitals correspond to chemically soft regions. We combine the orbital overlap distance with electrostatic potentials to quantify the hardness and electrophilicity of different sites in heterocyclic aromatic compounds. Results are compared to known experimental trends in aromatic reactivity
CHEM2019LE4831 CHEM
Formation of Platinum Nanocrystals on Silicon Nanotubes and in vitro Anti-cancer Activity of the composites
Type: Graduate
Author(s):
Nguyen Le
Chemistry & Biochemistry
Advisor(s):
Jeffery Coffer
Chemistry & Biochemistry
Giridhar Akkaraju
Biology
Location: Session: 2; 1st Floor; Table Number: 3

(Presentation is private)The semiconductor Silicon (Si) remains a significant material in the electronic device and photovoltaic industries [1]. Especially, nanostructured forms of Si with a porous morphology (pSi) exhibit interesting properties which can be controlled via modulating pore structure and surface chemistry [1]. Recently, synthesis of a unique one-dimensional form of Si, namely nanotubes, with tunable structure (shell thickness, length, inner diameter and porous morphology) has been demonstrated, thereby suggesting newly emerging applications [2]. For instance, recent works have indicated Si nanotubes (SiNTs) can efficiently serve as a reaction vessel for formation of organometal perovskite nanostructures and a template for superparamagnetic iron oxide (Fe3O4) loading [3], [4]. In an observation of dissolution of SiNTs with a porous morphology (pSiNTs), the material readily resorbed in buffered media at physiological conditions in a similar manner to bioactive nanostructured porous silicon, thereby implying potential therapeutic applications of this material [2].
In chemotherapy, platinum-based cancer drugs, such as cisplatin and carboplatin, are widely used as effective drugs against various types of cancer [5]. Interestingly, while elemental platinum nanoparticles (Pt NPs) have been well investigated in diverse catalytic processes, in recent years, Pt NPs have also been discovered as a potent anti-cancer agent in nanomedicine, implying the use of the nanodrug to counteract chemoresistance in some cancer cell lines [6], [7]. Recent reports have also indicated that enhanced cytotoxicity against selected cancer cell lines is ascribed to ultra-small Pt NPs, especially those with size less than 3 nm [7]. In this report, pSiNTs were investigated as a template for the formation of Pt NPs, and in vitro cytotoxicity of the composites was evaluated against HeLa cancer cells.
Regarding fabrication, pSiNTs with short lengths (~500 nm) and thin walls (~10 nm) were synthesized via a ZnO nanowire sacrificial template method. Based on a combination of characterization techniques [High resolution transmission electron microscopy (HR-TEM) and energy dispersive X-ray analysis (TEM-EDX)], it is suggested that pSiNTs surface functionalized with 3-aminopropyltriethoxysilane can facilitate formation of Pt nanocrystals (Pt NCs) with size ranging from 1-3 nm utilizing a K2PtCl4 precursor. By varying reaction conditions (concentration of Pt salt and incubation time), the amount of Pt NCs deposited on SiNTs can be sensitively tuned from 20 to 55 wt%. In terms of cytotoxicity evaluation of the composites against HeLa cells, cellular viability was assessed using CellTiter-Glo assays, which quantified the amount of ATP in metabolically active cells. Our findings suggest that Pt NCs-SiNTs composites were toxic to HeLa cells, and less than 50% cells were still viable after 3 days of treatment with the composites at doses of 35 μg/ml and 50 μg/ml. Results from caspase 3/7 assays also showed that caspase 3/7 level in cells treated with Pt NCs-SiNTs approximately ranged from 1.5 to 2-fold increase compared to cells without treatment, thereby suggesting apoptosis as the likely mechanism. In vitro cellular uptake studies analyzed by confocal microscopy also confirmed accumulation of the composites within the cytoplasm of the cells after the treatment, consistent with a “Trojan horse” mechanism in which high concentrations of Pt NCs are internalized within cells assisted by pSiNTs and subsequently released via dissolution of the nanotube matrix.
The studies presented herein describe a novel strategy to form and immobilize highly compact clusters of Pt NCs by using pSiNTs as a template. In terms of bio-relevant applications, in vitro studies provide new insights into the anti-cancer properties of the newly discovered composites in inducing apoptosis in HeLa cells, thereby providing significant potential uses of Pt NCs-SiNTs in cancer treatment. Further investigations into gene expression profile(s) may be necessary in order to clarify the impact of the composites on cell survival in terms of molecular mechanisms.
References
1. H. Santos, Porous Silicon for Biomedical Applications, Ed. Cambridge: Woodhead Publishing, (2014).
2. X. Huang, R. Gonzalez-Rodriguez, R. Rich, Z. Gryczynski and J. L. Coffer, Chem. Commun., 49, 5760 (2013).
3. R. Gonzalez-Rodriguez, N. Arad-Vosk, N. Rozenfeld, A. Sa'ar and J. L. Coffer, Small, 12(33), (2016).
4. P. Granitzer, K. Rumpf, R. Gonzalez, J. Coffer, M. Reissner, Nanoscale Res. Lett., 9, 413 (2014).
5. T. C. Johnstone, K. Suntharalingam and S. J. Lippard, Chem. Rev., 116 (5), 3436–3486, (2016).
6. X. Li, G. Li, W. Zang, L. Wang and X. Zhang, Catal. Sci. Technol., 4, 3290-3297 (2014).
7. H. Xia, F. Li, X. Hu, W. Park, S. Wang, Y. Jang, Y. Du, S. Baik, S. Cho, T. Kang, D. Kim, D. Ling, K. M. Hui and T. Hyeon, ACS Cent. Sci., 2, 802−811 (2016).
CHEM2019MEHMOOD38923 CHEM
An Orbital-overlap Scale for Solvent Hardness and Softness: Method and Application to Ionic Liquids
Type: Graduate
Author(s):
Arshad Mehmood
Chemistry & Biochemistry
Advisor(s):
Benjamin G. Janesko
Chemistry & Biochemistry
Location: Session: 1; 2nd Floor; Table Number: 1

View PresentationThe chemical hardness of a solvent can play a decisive role in solubility and reactivity in solution. Several empirical scales of solvent softness have been proposed. We explore whether computed properties of solvent molecules can reproduce these empirical scales. Our "orbital overlap distance" quantifying the size of orbitals at a molecule's surface effectively reproduces the Marcus μ-scale of solvent softness. The orbital overlap distance predicts that the surfaces of chemically hard solvent molecules is dominated by compact orbitals possessing a small orbital overlap distance. In contrast, the surface of chemically soft solvent molecules has a larger contribution from diffuse orbitals and a larger orbital overlap distance. Other "conceptual density functional theory" descriptors, including the global hardness and electronegativity, can also reproduce empirical solvent scales. We further introduce a "solvent versatility" RMSD Dsurf scale quantifying variations in the surface orbital overlap distance. "Good" solvents such as DMSO, which combine chemically "hard" and "soft" sites within a single molecule, possess a large RMSD Dsurf. We conclude by applying this approach to predict the Marcus μ-parameters for widely-used ionic liquids and ionic liquid - cosolvent systems.
CHEM2019MEKHAIL60535 CHEM
Functional groups effect on the electronics of macrocyclic pyridinophane
Type: Graduate
Author(s):
Magy Mekhail
Chemistry & Biochemistry
Advisor(s):
Kayla Green
Chemistry & Biochemistry
Location: Session: 2; 3rd Floor; Table Number: 8

View PresentationThe use of macrocyclic pyridinophane has been growing in the fields of bioinorganic modeling, catalysis and imaging. However, the functionalization of the pyridine has not been fully explored. Therefore, the Green Research Group we produce a series of 12-membered tetra-aza N-heterocyclic amines, derived from pyclen with different functional groups substituted at the para position. Using Hammett plot analysis, X-ray diffraction, electrochemistry and C-C coupling catalytic results, we aim to understand the impact of these functional groups on the donating) of the ligand. From the Hammett plot results we predict how other functional groups will affect the electronics and reveal whether the resonance or inductive effects will mitigate the coordination environment.
The use of macrocyclic pyridinophane has been growing in the fields of bioinorganic complexes modeling, catalysis, and imaging. However, the functionalization of the pyridine has not been fully explored. Therefore, the Green Research Group produced a series of 12-membered tetra-aza macrocycles derived from pyclen with different functional groups substituted at the para position. Using Hammett plot analysis, X-ray diffraction, electrochemistry, and C-C coupling catalytic results, we aim to understand the impact of these functional groups on the donor ability of each ligand. From the Hammett plot results we hope to predict how other functional groups will affect the electronics and reveal whether the resonance or inductive effects will mitigate the coordination environment and reactivity of each complex.
CHEM2019MONTOYA22550 CHEM
Steps Towards the Total Synthesis of Amaryllidaceae Alkaloids
Type: Graduate
Author(s):
Adam Montoya
Chemistry & Biochemistry
Advisor(s):
David Minter
Chemistry & Biochemistry
Location: Session: 1; 3rd Floor; Table Number: 6

(Presentation is private)Phenanthridone-type alkaloids isolated from certain plants of the Amaryllidaceae family are of interest due to their pharmaceutically active nature. The compounds are commonly used in research concerning cancer, Alzheimer’s disease and other human illnesses. One of the main hindrances to such research is the limited availability of many of these compounds. The Minter group is interested in the development of procedures for synthesizing such alkaloids in a cost-effective and time efficient manner, while at the same time maintaining fair to excellent yields.
Techniques toward the synthesis of natural products of the Phenanthridone type are presented herein. Manipulations were tested and optimized on a model system in order to save both time and funds while developing a synthetic pathway to be utilized in the formation of more complex compounds. Setbacks such as controlling the stereochemistry of a tetra-substituted double bond reduction have been encountered. However, adjustments are being made to avoid such difficulties in the future. Ideally, the proposed scheme will ultimately allow for the synthesis of multiple phenanthridone analogs.
CHEM2019NEWELL46942 CHEM
Preparation of Clickable Monomers Compatible with Automated PNA Synthesis
Type: Undergraduate
Author(s):
Grace Newell
Chemistry & Biochemistry
Advisor(s):
Jean-Luc Montchamp
Chemistry & Biochemistry
Location: Session: 2; Basement; Table Number: 1

View PresentationPeptide nucleic acids (PNA) are artificially synthesized monomers or polymers that mimic DNA or RNA sequences. Due to their stability in biological conditions and their ability to bind complementary to DNA or RNA, PNAs have potential medicinal value since they can be used to block processes like replication or protein synthesis. Though most PNAs are commercially synthesized, the goal of this project was to begin the synthesis with propargyl bromide. This would allow the final monomer to have a propargyl group which allows functional groups (like a polyamine tail, fluorescent tag, or alkylating group) to be added at the end or any time throughout the synthesis. The PNA monomer will be made with all four DNA bases (thymine, cytosine, adenine, and guanidine) attached. Another importance of this PNA monomer is its ability to undergo click reactions to create a PNA oligomer. Click chemistry is a chemical reaction that uses copper-catalyzed coupling to combine an azide with an alkyne. The ability to use click chemistry is vital since it can be done in biological conditions, has an excellent yield with few byproducts, and is relatively quick to perform. In conclusion, this project is useful since these PNA sequences can be used to modulate processes and treat a variety of diseases while having the ability to add functional groups to track the PNA oligomer.
CHEM2019NIEBUHR27253 CHEM
Functional Modifications and Electronic Influences on Macrocyclic Tetra-aza Copper (II) Complexes
Type: Undergraduate
Author(s):
Brian Niebuhr
Chemistry & Biochemistry
Marianne Burnett
Chemistry & Biochemistry
Advisor(s):
Kayla Green
Chemistry & Biochemistry
Location: Session: 2; Basement; Table Number: 2

View PresentationA library of novel pyridinophane tetra-aza macrocyclic molecules derived from 1,4,7,10-tetraaza-2,6-pyridinophane (pyclen) capable of chelating biologically relevant metal ions have been synthesized. Applications of these types of molecules currently being pursued are 1) therapeutic, focusing on radical scavenging and metal chelation and 2) diagnostic, focusing on magnetic resonance imaging (MRI) contrast agents when complexed with specific metal ions. Despite wide interest in these molecules, a full study of the electronic effects imparted by substitution to the pyridyl moiety and the subsequent impact on the metal center has not yet been conducted. The objective of the present study is to characterize metal complexes of four, new tetra-aza macrocyclic metal chelating molecules. The pyridyl functional groups studied include: A) unmodified pyridyl (L1), B) 14-hydroxyl (L2), C) 14-nitrile (L3), and D) 14--nitro (L4) modified pyclen structures. Procedures for metal ion chelation with copper (II) ion, followed by characterization and analysis of the electronic environments of each are presented.
CHEM2019PHAM64839 CHEM
Optimization of Tin(IV) Oxide Particles For Improved Performance of Dye-Sensitized Solar Cell
Type: Undergraduate
Author(s):
Bach Pham
Chemistry & Biochemistry
Advisor(s):
Benjamin Sherman
Chemistry & Biochemistry
Location: Session: 1; 3rd Floor; Table Number: 10

View PresentationThe dye-sensitized solar cells (DSSCs) are a possible alternative tool to harvest solar energy instead of the traditional silicon-based solar cells. DSSCs offer various advantages, such as good energy conversion efficiencies in low-light condition, simple fabrication, low cost, and the ability to modify key properties of the solar cell such as the absorbance wavelengths. We are interested in developing new types of semiconductor supports for use in DSSCs based on tin(IV) oxide nanoparticles (NPs). Tin(IV) oxide offers a wide band gap and higher electron mobility as compared with the more widely used titanium dioxide. In this study, two morphologies of tin(IV) oxide, spherical and flower-like NPs, are synthesized. These two types of tin(IV) oxide NPs and mixtures of both at various ratios are used to fabricate DSSCs. We find that nanoflowers usually give the cells higher open circuit voltages but with lower photocurrent. Nanospheres give much higher photocurrent but with lower open circuit voltage. A mixture that has a 1:2 molar ratio of nanoflowers and nanospheres gave the best performance in terms of photocurrent and voltage. Furthermore, we are investigating the effect of a deposited layer of titanium(IV) oxide on top of the tin(IV) oxide to further enhance the photoperformace of the solar cells.
CHEM2019SCHMITT50258 CHEM
The Synthesis of Amaryllidaceae Alkaloid Analogs
Type: Undergraduate
Author(s):
Nate Schmitt
Chemistry & Biochemistry
Adam Montoya
Chemistry & Biochemistry
Advisor(s):
David Minter
Chemistry & Biochemistry
Location: Session: 2; 1st Floor; Table Number: 4

View PresentationAmaryllidaceae isoquinoline alkaloids as well as their analogs have long been of interest as lead compounds in drug discovery due to their range of biological activity. Many of these alkaloids are cytotoxic anti-tumor agents. Moreover, there have also been studies showing the effectiveness of these molecules against yellow fever and other diseases caused by RNA- containing flaviviruses. The study of these compounds as pharmaceutical agents is hampered by their low natural abundance, which necessitates the development of laboratory syntheses of these alkaloids and their analogs.
This project focuses on the total syntheses of the Pancratistatin-type natural products that contain the phenanthridone ring system. In stage one, model systems are being investigated to develop the methodology required to introduce requisite functionality found in natural systems. Previous research from this laboratory gives the basic phenanthridone skeleton with several different functional groups, but there are no reported methods for converting these functions into polyhydroxycyclohexenes with stereochemical control. Two of the problems under investigation involve the ring expansion of a spiro ring containing an epoxide and the production of a specific trihydroxycyclohexene with control of stereochemistry. In stage two, a specific phenanthridone alkaloid will be targeted for total synthesis that uses the new methodology developed in stage one.
CHEM2019SCHWARTZ27386 CHEM
Synthesis and Characterization of N,N,N,-Copper Pincer Complexes
Type: Undergraduate
Author(s):
Timothy Schwartz
Chemistry & Biochemistry
Marianne Burnett
Chemistry & Biochemistry
Akop Yepremyam
Chemistry & Biochemistry
Advisor(s):
Kayla Green
Chemistry & Biochemistry
Location: Session: 2; 1st Floor; Table Number: 2

View PresentationOrganometallic catalysts are useful in many organic reactions by exploiting the Lewis acidity of the metal complex. Most catalysts available rely on precious metals like platinum and rhenium. These catalysts pose a financial and environmental barrier to many scientists. Thus, there is a need for catalysts that use less expensive and toxic metals, such as copper. A library of copper catalysts with different electronic functionalities have been synthesized and characterized by cyclic voltammetry, UV-VIS, NMR, and X-ray crystallography. It was found that the complexes with electron donating groups better stabilize the copper center, when compared to the complexes with electron withdrawing groups. However, the planar characteristics of each ligand makes them unsuitable candidates for copper catalysis because they cannot bind to the tetrahedral geometry of reduced copper. This work warrants the complexation of these ligands with other metals, like nickel or cobalt, to determine their viability as applicable organometallic catalysts.
CHEM2019SHARMA21186 CHEM
Exploring Cyanuric Chloride Chemistry to Synthesize Macrocycles of Different Sizes
Type: Graduate
Author(s):
Vishal Sharma
Chemistry & Biochemistry
Advisor(s):
Eric Simanek
Chemistry & Biochemistry
Location: Session: 1; 3rd Floor; Table Number: 4

View PresentationIn chemistry, cyclic compounds of twelve or more atoms are considered macrocycles. Many bioactive, natural products containing macrocycles have been isolated and synthesized. Still, construction of macrocycles is usually considered a challenging step in their synthesis. Here, a route to different-sized macrocycles is described. These macrocycles arise from spontaneous cyclization of two identical subunits comprising a central triazine displaying both a masked aldehyde and hydrazine group. The aldehyde portion is presented on a linker that can comprise varying number of carbons. By varying this linker, macrocycles of 22, 24, and 26 atoms have been prepared. Future study focuses on probing macrocycle size with increasingly larger linkers.